

A Battery is an electrical item that is used in many day-to-day items like Laptops, phones, toys, mechanical tools, etc. Now let’s talk about how the battery was invented!
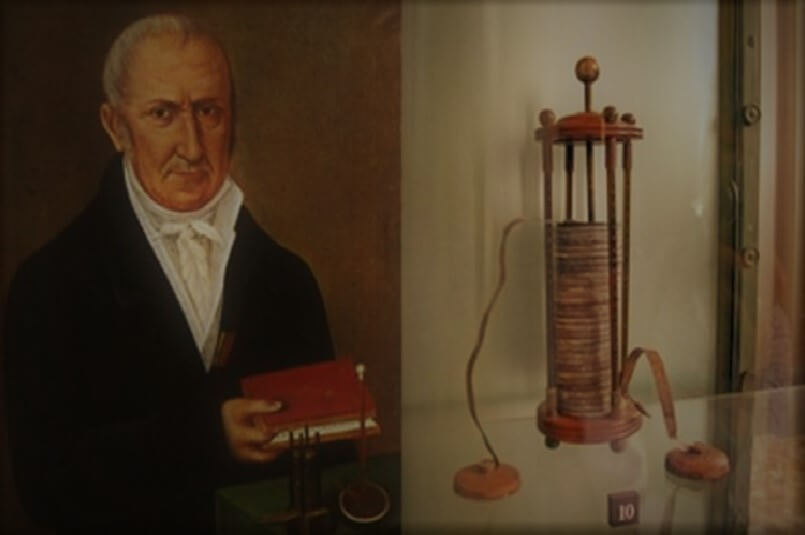
Alessandro Volta was an Italian physicist who invented the first working battery. He placed Zinc and Copper blocks separated by an electrolyte on a metal stand. Alessandro connected this battery with light and surprisingly, it worked! You can try his experiment by placing zinc and copper strips in 3 lemons and connecting this to light. It is a very educational experiment that you can do to learn more about Alessandro’s Invention! If you want to learn more about his invention, you could visit Tempio Voltiano in Como, Italy! Since his invention, the battery was modified and his invention made all of our lives very different!
Now, what’s inside a battery? A battery contains a liquid electrolyte that contains Sodium Hydroxide. It also has electrons that go 400+ times from the battery to the power source. After these many rotations, the battery dies. The electrolyte prevents the battery from shorting out and it is the main part of a battery that generates electricity. The battery also contains zinc and copper like how Alessandro put on his battery. So this is how a typical battery works!
I really hope that we soon get some green environmentally friendly batteries that do not have chemicals to make our earth a better place to live for our future generations to come.
Author: Sri Nihal Tammana

© copyright 2022 by Recycle My Battery